RPM™ Reinforced PTFE Mesh

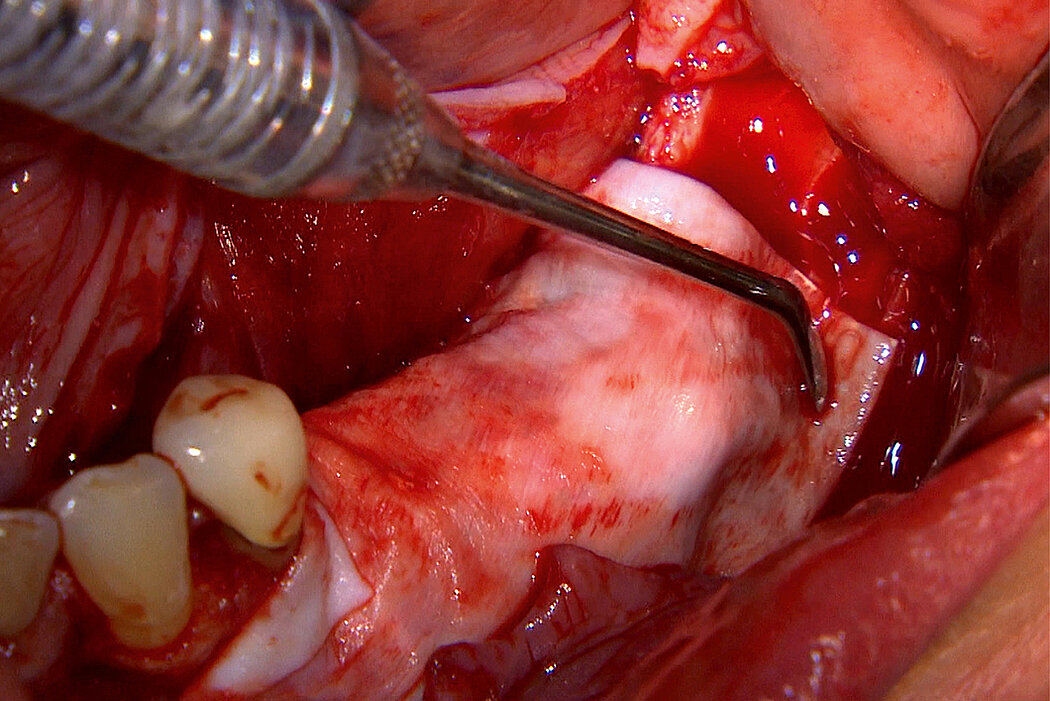
Vertical augmentation with RPMTM
User benefits
Reinforce, Revascularize, Regenerate
Have you ever wanted to take advantage of a form-stable barrier membrane that allows for better vascularization of the underlying bone graft, as like with collagen membranes?
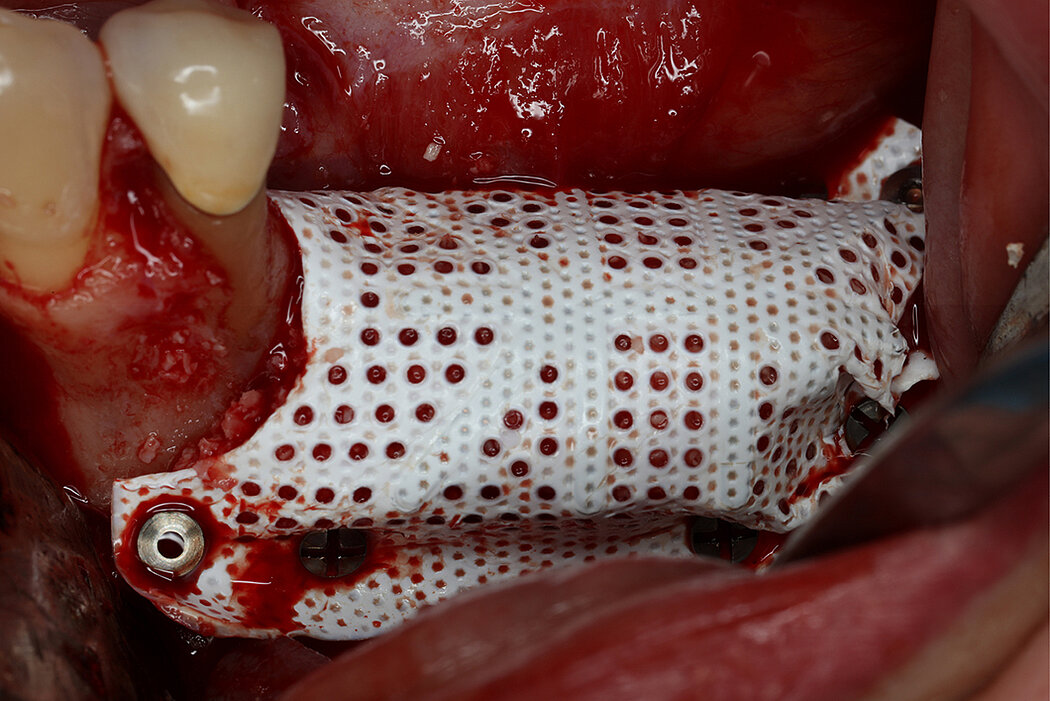
Titanium-reinforced polytetrafluoroethylene (PTFE) membranes are well documented off-the-shelf solution for vertical alveolar ridge augmentation in oral and maxillofacial bone regeneration1. They provide stability through the integrated titanium structure and provide a secluded space for undisturbed bone formation. However, classical PTFE membranes are occlusive membranes that do not allow vascularization from the periosteum. Early vascularization plays a central role in guided bone regeneration as angiogenesis is fundamental to new bone formation2,3,4. The novel Re-inforced PTFE Mesh5, RPMTM, is characterized by perforations that allow vascularization from the periosteum.
- Circular perforations of 660 microns (0.66mm) -> allowing proper graft vascularization from periosteum to bone graft
- Titanium frame maintains space -> essential for vertical bone augmentation6
- PTFE allows easy trimming and adaptation -> thickness of 250 microns, the most inert & stable polymer in the biological system6
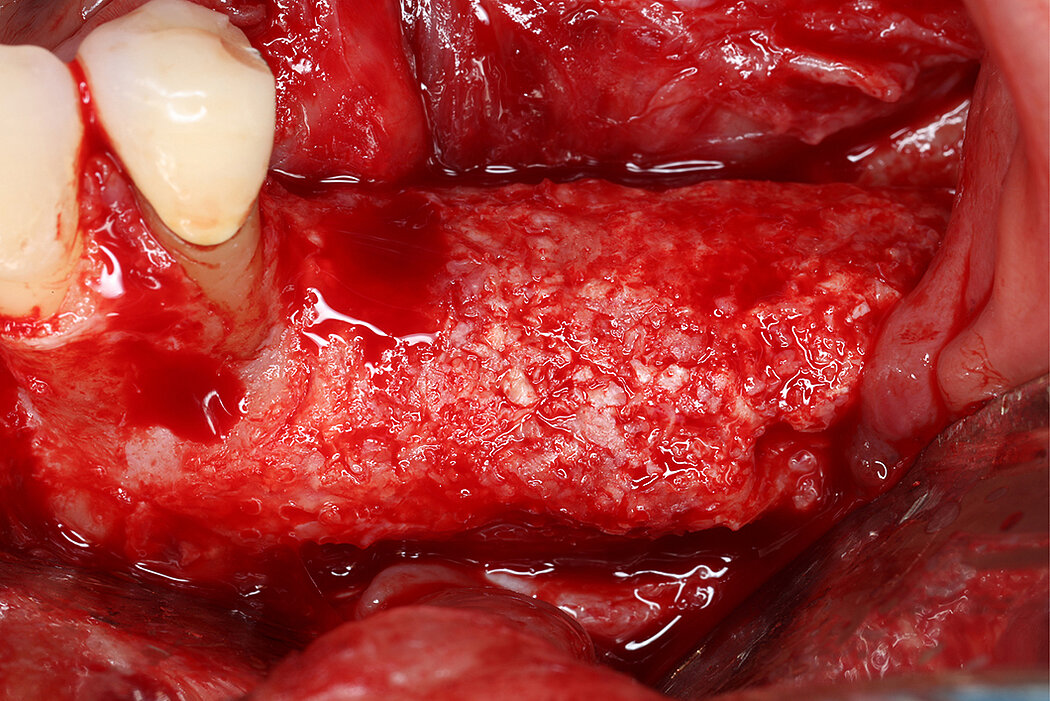
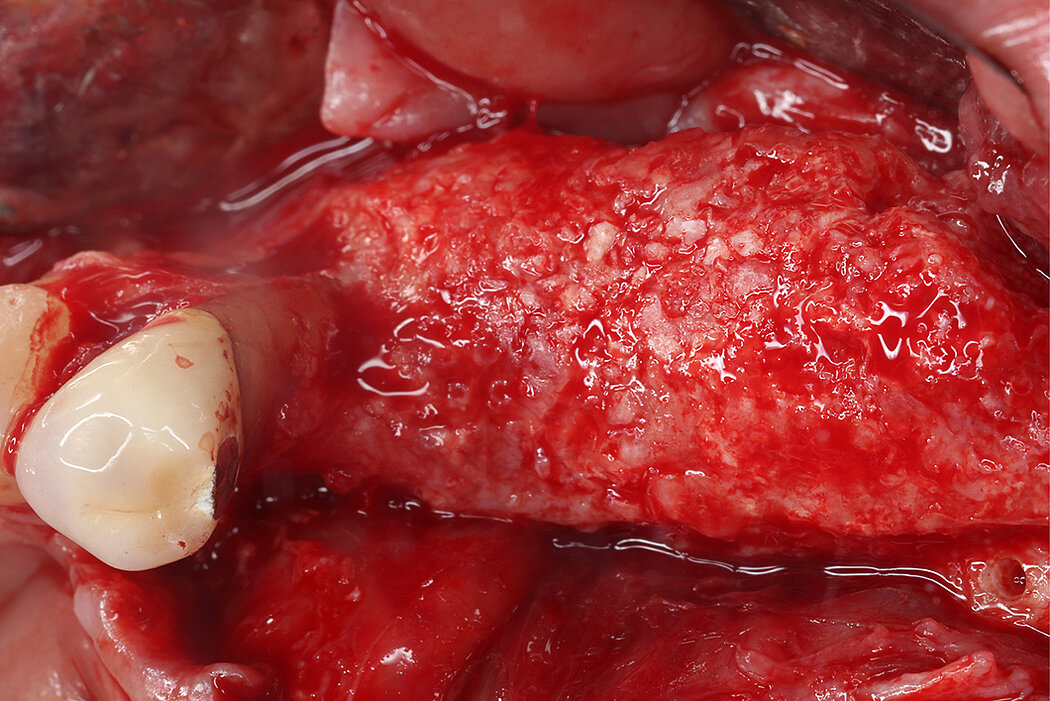
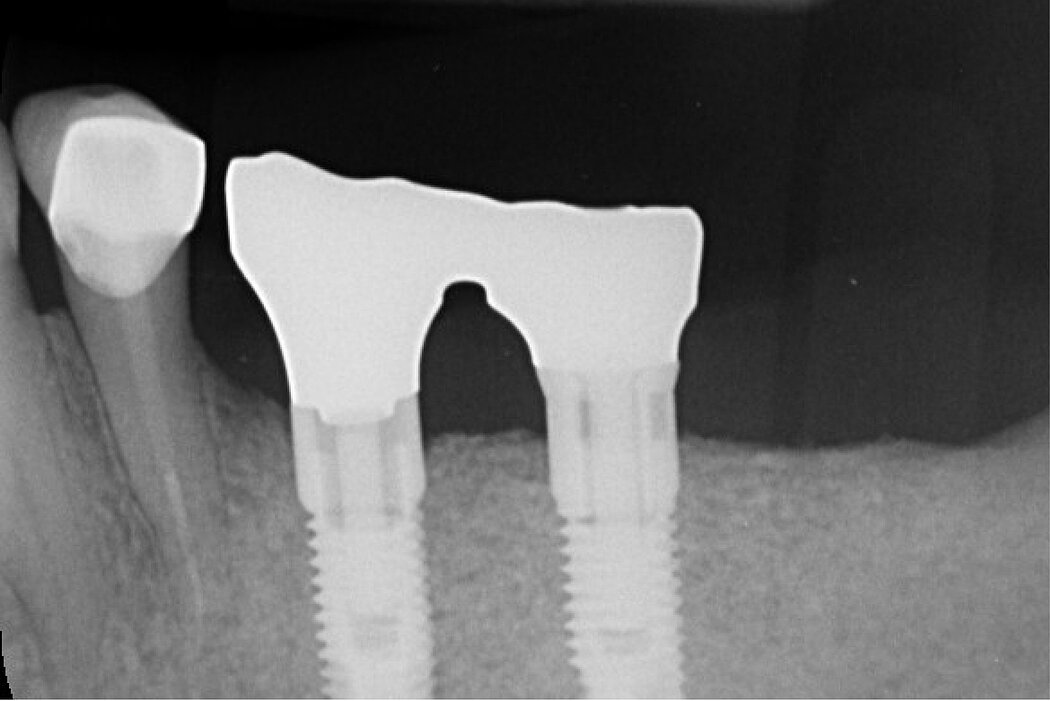
RPMTM combines the benefits of a stable scaffold and a PTFE barrier membrane, whilst allowing optimal supply of the bone graft through vascularisation from the periosteum to obtain vital bone. The concept and design of RPMTM was co-developed with Prof. Istvan Urban, Hungary/USA and reflects the same principles as the Sausage TechniqueTM (7), but for vertical alveolar ridge augmentation8.
References:
- Urban IA, et al: Int J Oral Maxillofac Implants 2009, 24 (3), 502-10
- Schwarz F et al. Clin. Oral Implants Res. 2008;19(4): 402-415. (Pre-clinical study)
- Rothamel D et al. Clin. Oral Implants Res. 2005; 16(3): 369-378. (Pre-clinical study)
- Perelman-Karmon M et al. Int J Periodontics Restorative Dent. 2012 Aug;32(4):459-65. (Clinical study)
- RPMTM is manufactured by Osteogenic Biomedical, Lubbock, Texas, USA
- Elgali I, et al: Eur J Oral Sci 2017; 125 (5), 315-337
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299-307.
- Urban IA, et al.: Int J Oral Maxillofac Implants 2014; 29(1): 185-93
- Urban IA, et al.: J Periodontics Restorative Dent 2017; 37(5):639-645 (clinical study) – review format of this reference
- Buser D et al., J Dent Res. 2013 Dec;92(12 Suppl):176S–82S (Clinical study).
- Urban IA et al., Int J Periodontics Restorative Dent 2015, 35(5):613-623 (clinical study) – review format of this reference
- Becker J et al. Clin Oral Implants Res. 2009; 20(7):742-749. (Clinical study)
- Tal H et al. Clin Oral Implants Res. 2008; 19(3) : 295-302. (Clinical study)
- Urban IA, et al Int J Periodontics Restorative Dent 2016, 36(3):329-337 (clinical study) review format of this reference
RPMTM has distinct configurations:
- Rectangular shapes
- With predefined fixation points distinctly outside the defect area
- For interproximal positioning
Selected shapes within the rectangular and interproximal portfolio are especially designed for the use in the mandible. The mandibular lingual side has mylohyoid muscle and vital anatomical landmarks9. Those shapes are characterized by a non-perforated area facing the lingual side and allow easy removal.
Bibliography:
- Urban IA, et al: Int J Oral Maxillofac Implants 2009, 24 (3), 502-10
- Schwarz F et al. Clin. Oral Implants Res. 2008;19(4): 402-415. (Pre-clinical study)
- Rothamel D et al. Clin. Oral Implants Res. 2005; 16(3): 369-378. (Pre-clinical study)
- Perelman-Karmon M et al. Int J Periodontics Restorative Dent. 2012 Aug;32(4):459-65. (Clinical study)
- RPMTM is manufactured by Osteogenic Biomedical, Lubbock, Texas, USA
- Elgali I, et al: Eur J Oral Sci 2017; 125 (5), 315-337
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299-307.
- Urban IA, et al.: Int J Oral Maxillofac Implants 2014; 29(1): 185-93
- Urban IA, et al.: J Periodontics Restorative Dent 2017; 37(5):639-645 (clinical study)
- Buser D et al., J Dent Res. 2013 Dec;92(12 Suppl):176S–82S (Clinical study).
- Urban IA et al., Int J Periodontics Restorative Dent 2015, 35(5):613-623 (clinical study)
- Becker J et al. Clin Oral Implants Res. 2009; 20(7):742-749. (Clinical study)
- Tal H et al. Clin Oral Implants Res. 2008; 19(3) : 295-302. (Clinical study)
- Urban IA, et al Int J Periodontics Restorative Dent 2016, 36(3):329-337 (clinical study)
Application
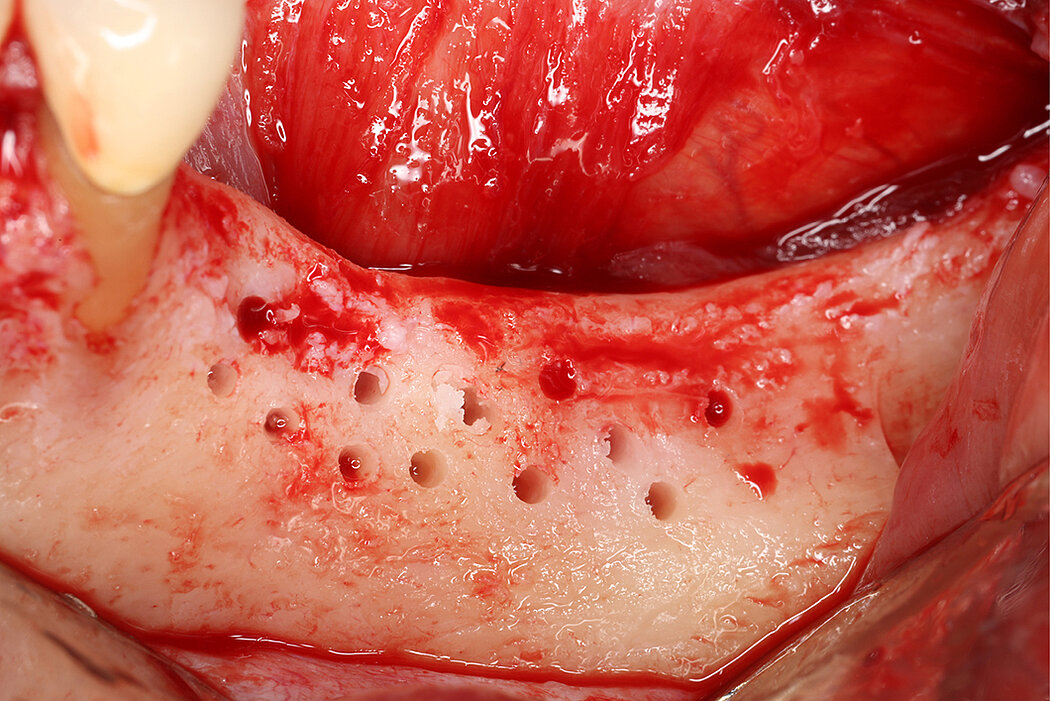
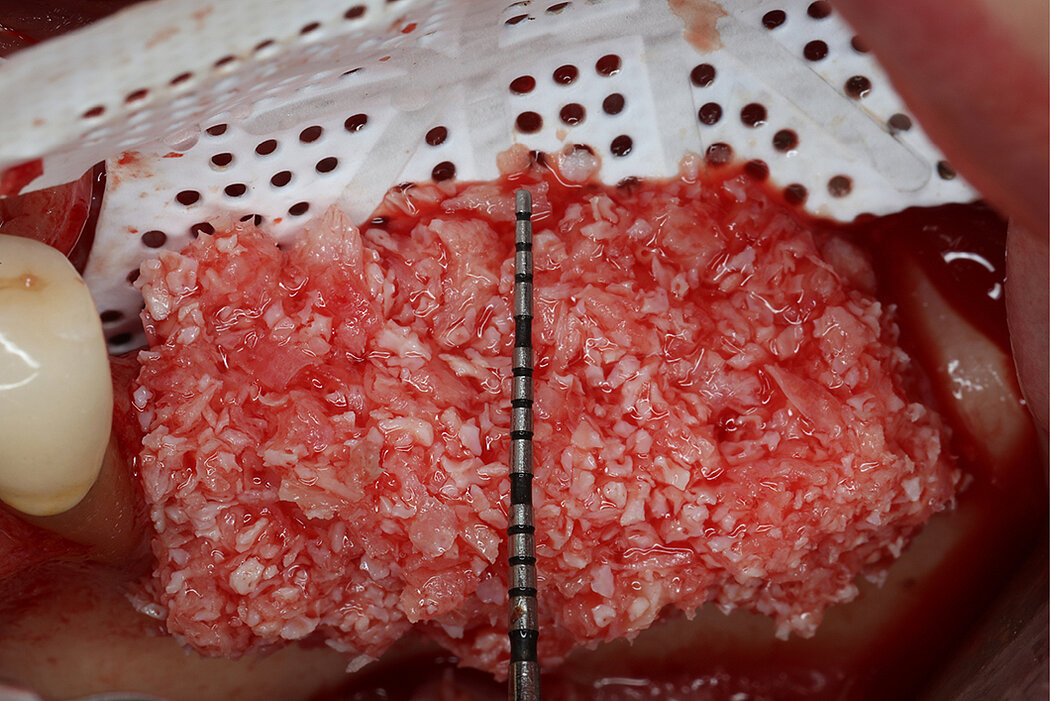
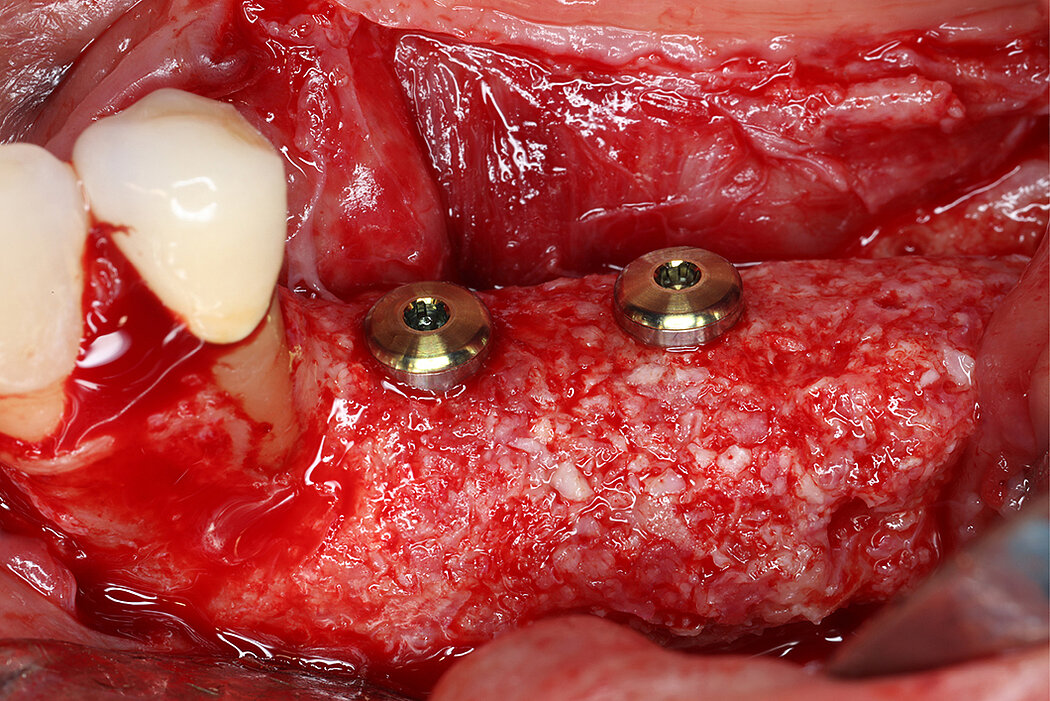
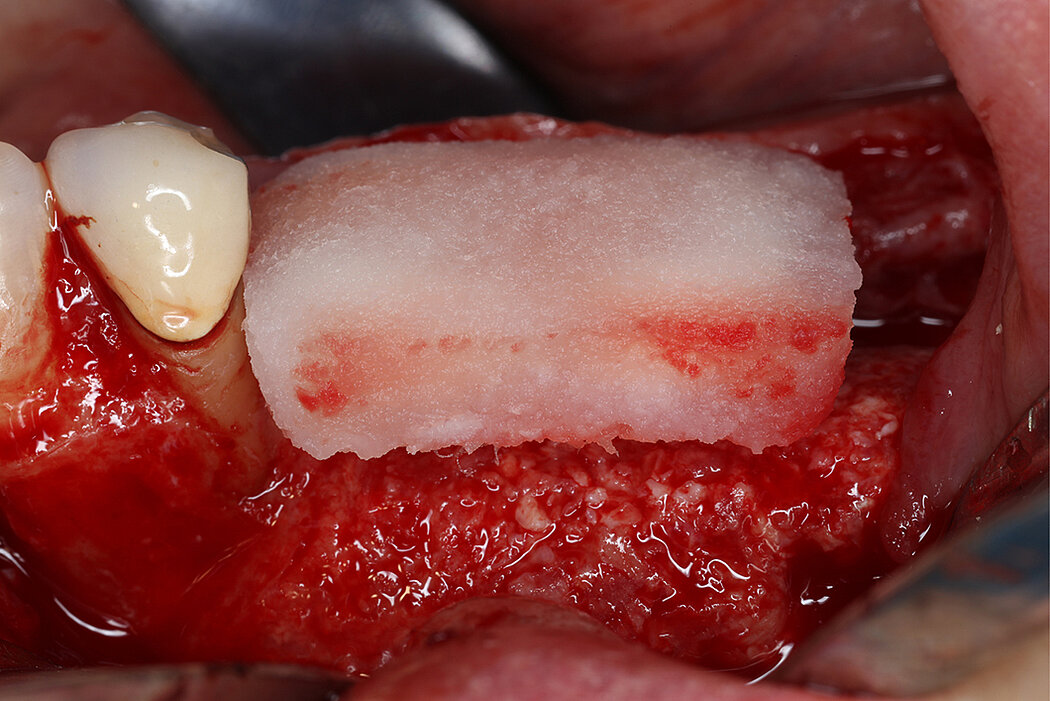
Re-inforced PTFE Mesh, RPMTM, can be easily trimmed, adapted, and fixed with pins. RPMTM offers a high stability and the secluded space can be augmented with a mixture of Geistlich Bio-Oss® and autologous bone. Geistlich Bio-Oss® provides long-term volume stability10, and autologous bone contain the required osteoinductive properties for vertical augmentation11. The autologous particulated bone graft can be harvested from the mandibular ramus using a bone scraper device9,11.
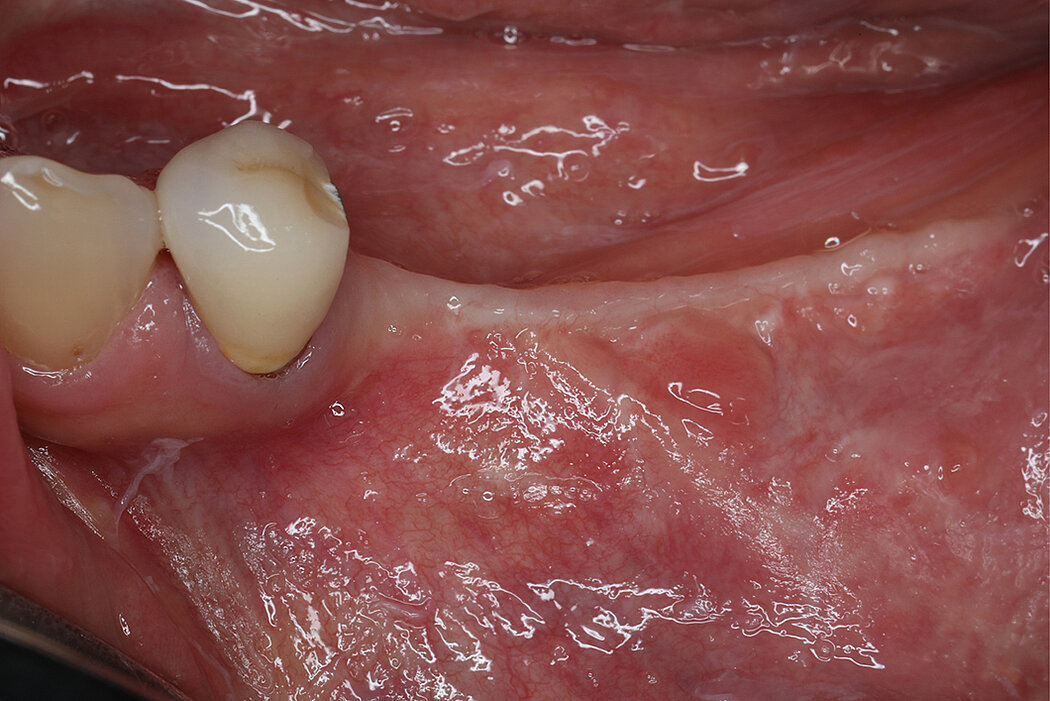
PTFE membranes have a long-standing history as barrier membranes and are highly biocompatible6. Collagen membranes, such as Geistlich Bio-Gide®, have been shown beneficial for uneventful soft tissue healing12,13 in guided bone regeneration (GBR) procedures resulting in low complication rates12. Thus, potential exposures can be anticipated by covering non-resorbable mesh with Geistlich Bio-Gide® to get a better soft tissue healing12,13. Moreover, both, Geistlich Bio-Gide® and RPMTM follow the concept of biofunctional membranes that allow early vascularization of the graft.
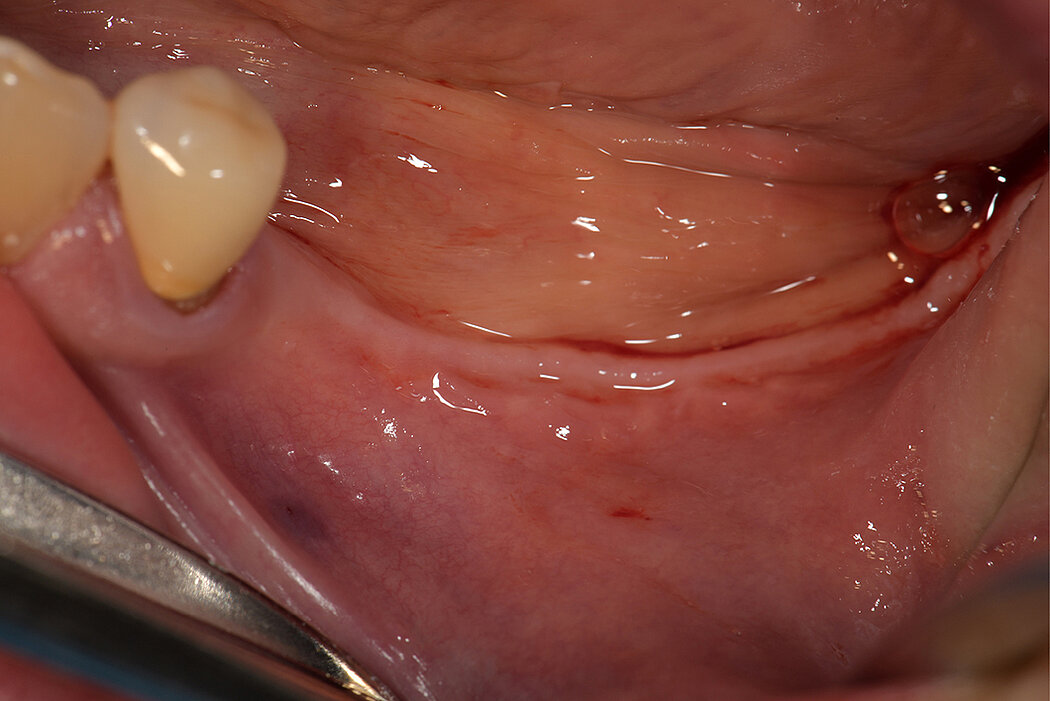
As for all augmentative procedures, soft tissue management is key9,14. This especially comprises:
- Preparation of soft tissues before, during, or after treatment to increase mucogingival thickness and keratinized tissue width
- Elevation of a full-thickness remote or papilla preservation flap
- Periosteal release to advance flap to achieve tension-free closure
- Primary soft tissue closure by horizontal mattress and single interrupted sutures