Chondro-Gide®

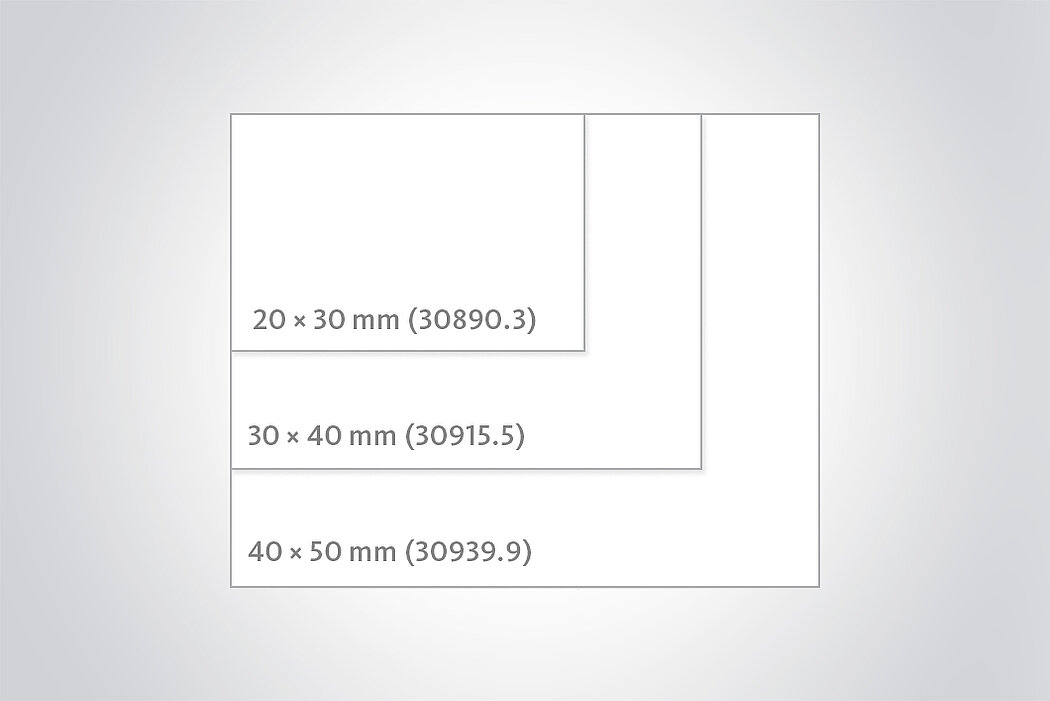
Chondro-Gide® is a bilayer collagen I/III membrane developed specifically for cartilage regeneration. Made from highly refined porcine collagen, it is produced in Switzerland following a rigorous quality assurance system to ensure its safety and quality.1
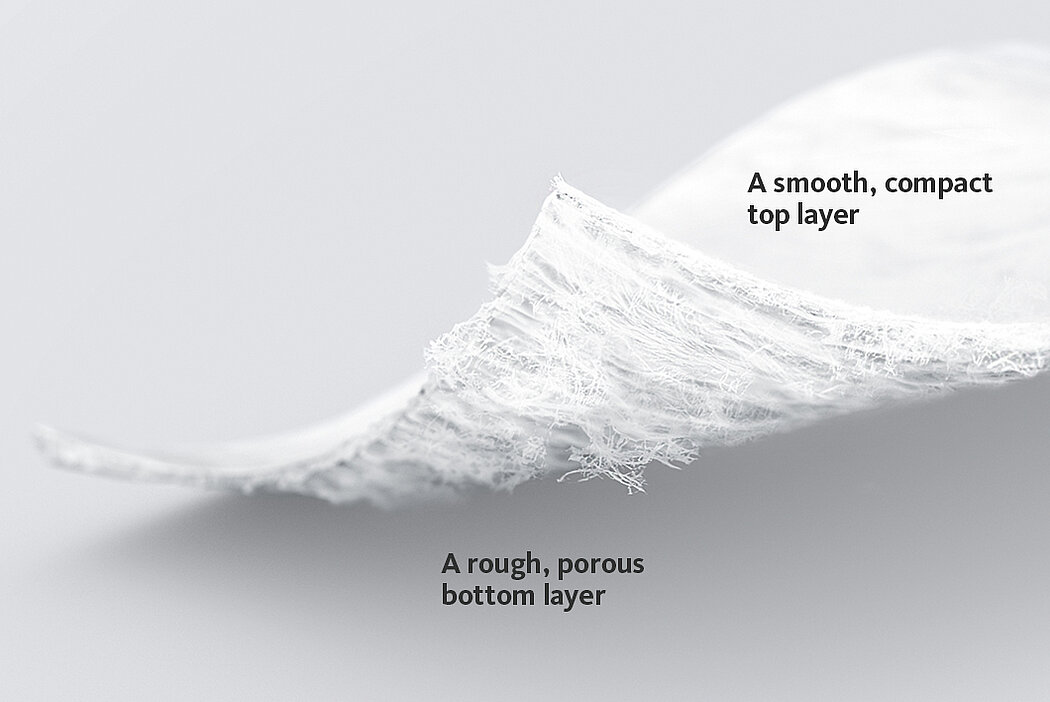
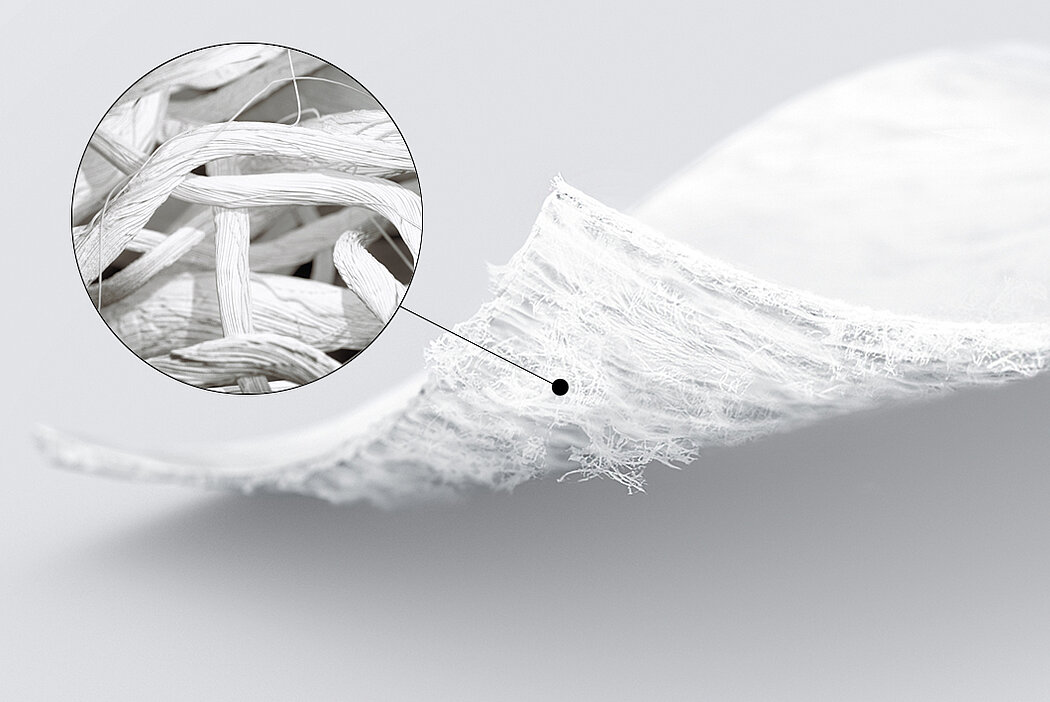
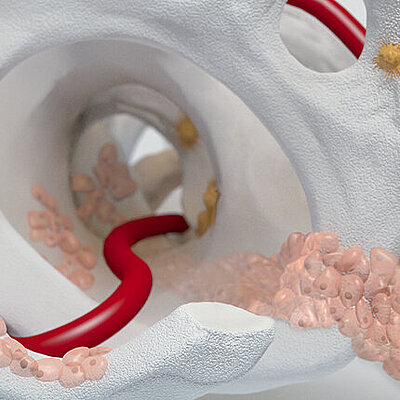
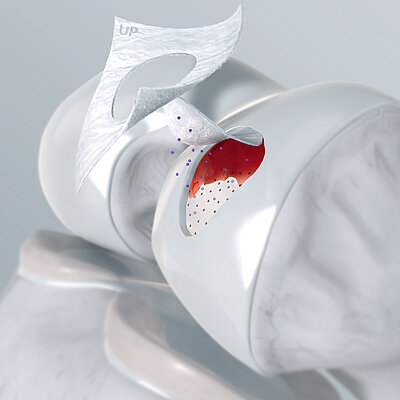
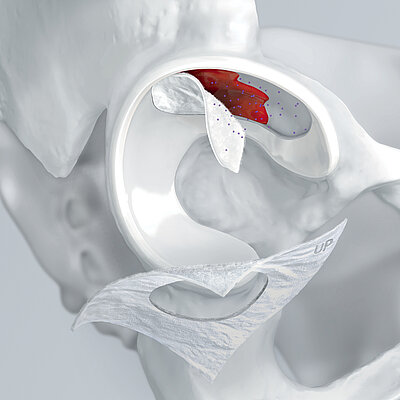
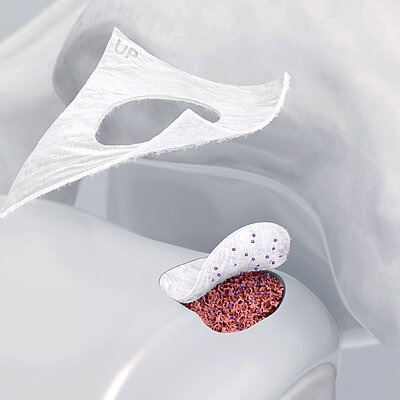
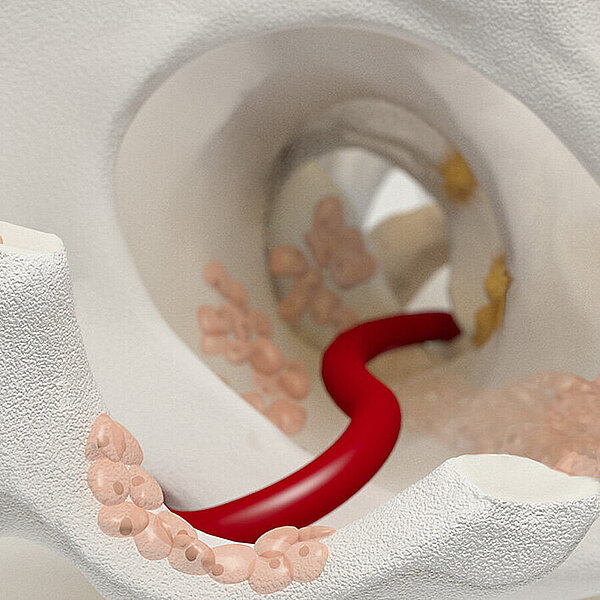
Chondro-Gide® works to leverage the body’s own healing potential to regenerate human cartilage. With a smooth, compact top layer, and a rough, porous bottom layer, its structure provides the conditions for cartilage regeneration.
References
- Geistlich Pharma AG data on file (Bench test)
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- VOLZ, M., et al. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop, Apr 2017, 41(4), 797-804. (Clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 63(5), 616-626. (Clinical study)
- GOMOLL, A.H., Surgical Management of Articular Cartilage Defects of the Knee. J Bone Joint Surg Am. 2010;92:2470-90 (Clinical study)
- MUMME, M., Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet, 2016, 388 (10055) 1985-1994. (Clinical study)
- FULCO, I., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet, Jul 26 2014, 84(9940), 337-346. (Clinical study)
- STEINWACHS, M.R., New Technique for Cell-Seeded Collagen Matrix-Supported Autologous Chondrocyte Transplantation, Arthroscopy: The Journal of Arthroscopic & Related Surgery Volume 25, Issue 2, February 2009, Pages 208-211 (Technical note)
- STEINWACHS, M. R., et al., "Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee." Cartilage 2019: 1947603519870846. (Review of clinical studies)
- CIEMNIEWSKA-GORZELA, K., et al.,Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: Clinical Effectiveness and Survivorship after a Minimum of 5 Years’Follow-Up. Cartilage. 2020 Jun 1 (Clinical study)
Benefits
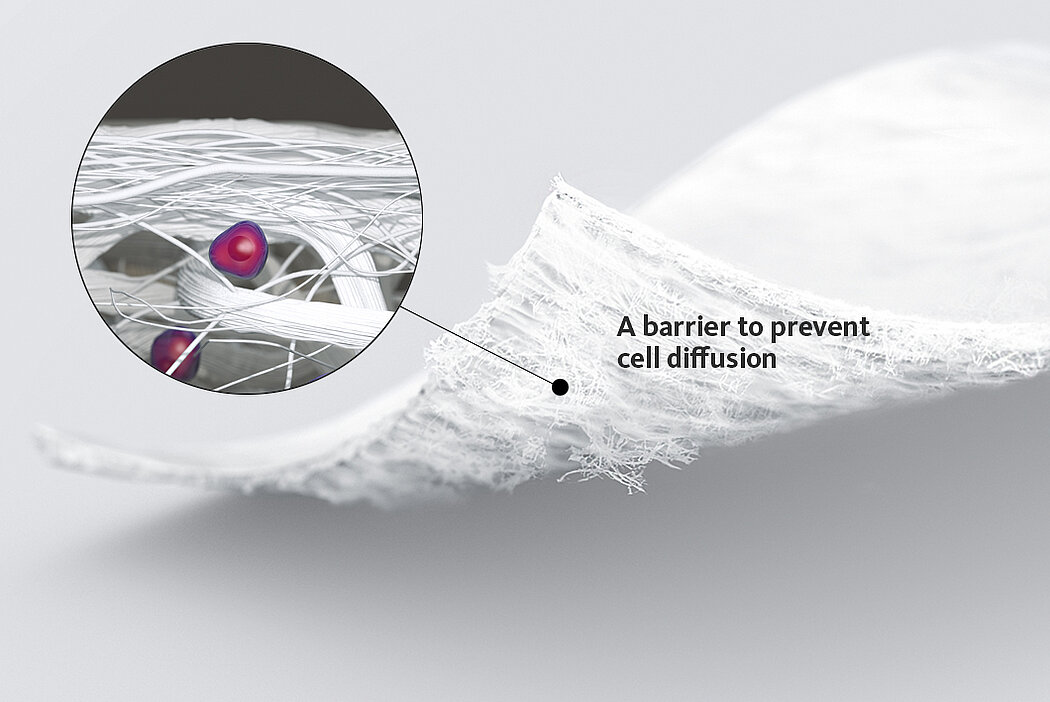
The success of Chondro-Gide® is backed by more than 10 years of clinical data2. With its specially designed bilayer structure, Chondro-Gide® provides a protective environment that fosters the growth of new cartilage.3,4
- Bio-derived, bilayer Collagen I/III membrane1
- Biocompatible and naturally resorbed1
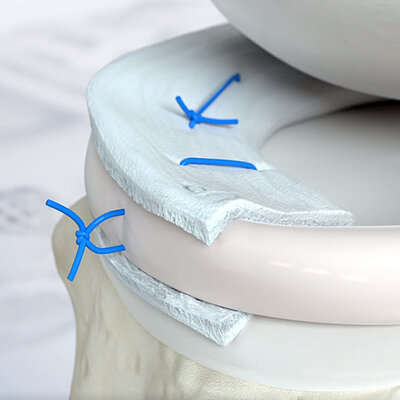
- Can be glued or sutured into place4
- Compatible with a range of tissue regeneration techniques5
- One-step procedure1
- Easy to handle: supple and tear-resistant1
- Ready for use off the shelf1
How Chondro-Gide® works
References:
- Geistlich Pharma AG data on file (Bench test)
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- VOLZ, M., et al. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop, Apr 2017, 41(4), 797-804. (Clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 63(5), 616-626. (Clinical study)
- GOMOLL, A.H., Surgical Management of Articular Cartilage Defects of the Knee. J Bone Joint Surg Am. 2010;92:2470-90 (Clinical study)
- MUMME, M., Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet, 2016, 388 (10055) 1985-1994. (Clinical study)
- FULCO, I., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet, Jul 26 2014, 84(9940), 337-346. (Clinical study)
- STEINWACHS, M.R., New Technique for Cell-Seeded Collagen Matrix-Supported Autologous Chondrocyte Transplantation, Arthroscopy: The Journal of Arthroscopic & Related Surgery Volume 25, Issue 2, February 2009, Pages 208-211 (Technical note)
- STEINWACHS, M. R., et al., "Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee." Cartilage 2019: 1947603519870846. (Review of clinical studies)
- CIEMNIEWSKA-GORZELA, K., et al.,Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: Clinical Effectiveness and Survivorship after a Minimum of 5 Years’Follow-Up. Cartilage. 2020 Jun 1 (Clinical study)
Surgical Techniques using Chondro-Gide®
Chondro-Gide® as a Carrier
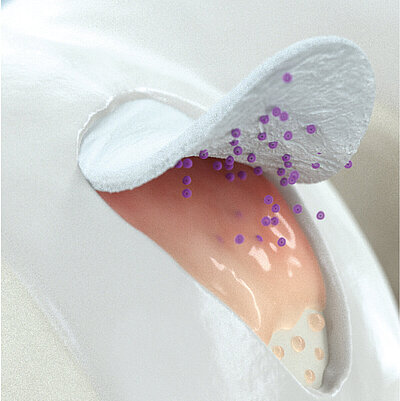
In autologous chondrocyte implantation techniques Chondro-Gide® serves as a carrier. It provides a 3D supportive matrix for adhesion and cell differentiation9. Cells can be seeded on the rough side of the bilayer membrane, while the smooth side prevents cell loss during the process.
Chondro-Gide® as a Cover
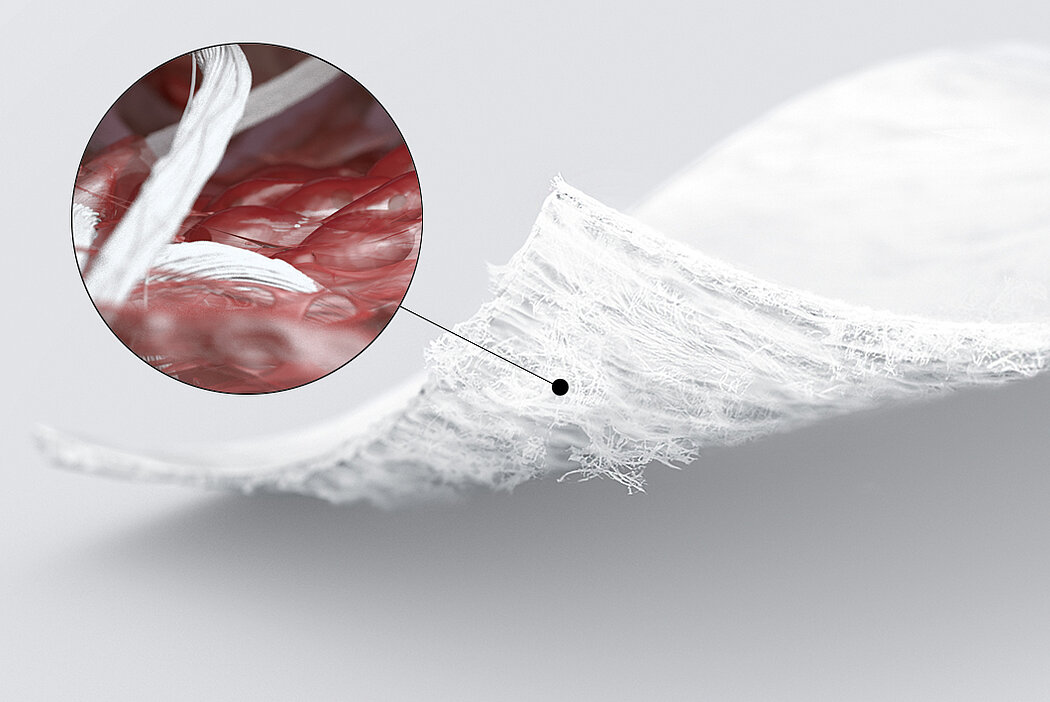
AMIC® (Autologous Matrix-Induced Chondrogenesis) is a single step, cost efficient and effective technique for treating cartilage defects. In AMIC®, Chondro-Gide® covers the defect and offers a protective environment for cell differentiation and the formation of new cartilage3,7,10. Learn more about AMIC® here.
Chondro-Gide® as a Wrap
AMMR® (Arthroscopic Matrix-Based Meniscus Repair) is a technique that was designed to preserve the meniscus. The membrane is wrapped around the damaged meniscus, the smooth side facing towards the joint cavity11. In one study, the room between the meniscus and the enveloping membrane was augmented with biological factors to enhance the repair process.
References
- Geistlich Pharma AG data on file (Bench test)
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- VOLZ, M., et al. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop, Apr 2017, 41(4), 797-804. (Clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 63(5), 616-626. (Clinical study)
- GOMOLL, A.H., Surgical Management of Articular Cartilage Defects of the Knee. J Bone Joint Surg Am. 2010;92:2470-90 (Clinical study)
- MUMME, M., Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet, 2016, 388 (10055) 1985-1994. (Clinical study)
- FULCO, I., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet, Jul 26 2014, 84(9940), 337-346. (Clinical study)
- STEINWACHS, M.R., New Technique for Cell-Seeded Collagen Matrix-Supported Autologous Chondrocyte Transplantation, Arthroscopy: The Journal of Arthroscopic & Related Surgery Volume 25, Issue 2, February 2009, Pages 208-211 (Technical note)
- STEINWACHS, M. R., et al., "Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee." Cartilage 2019: 1947603519870846. (Review of clinical studies)
- CIEMNIEWSKA-GORZELA, K., et al.,Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: Clinical Effectiveness and Survivorship after a Minimum of 5 Years’Follow-Up. Cartilage. 2020 Jun 1 (Clinical study)