Sinus Floor Elevation
Scientific background
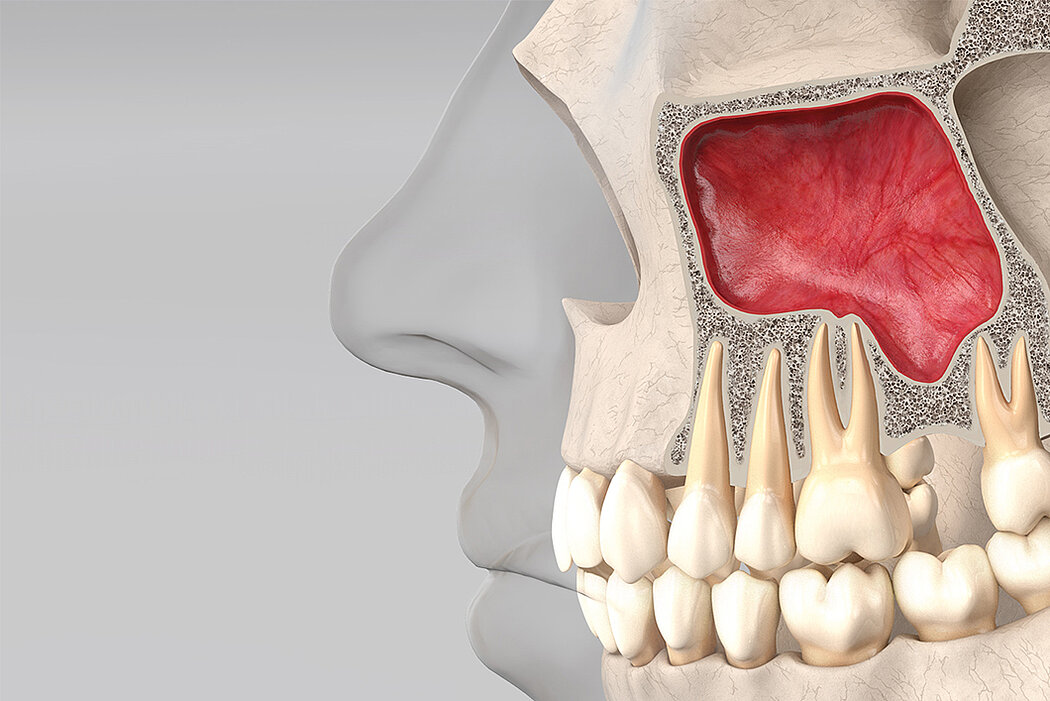
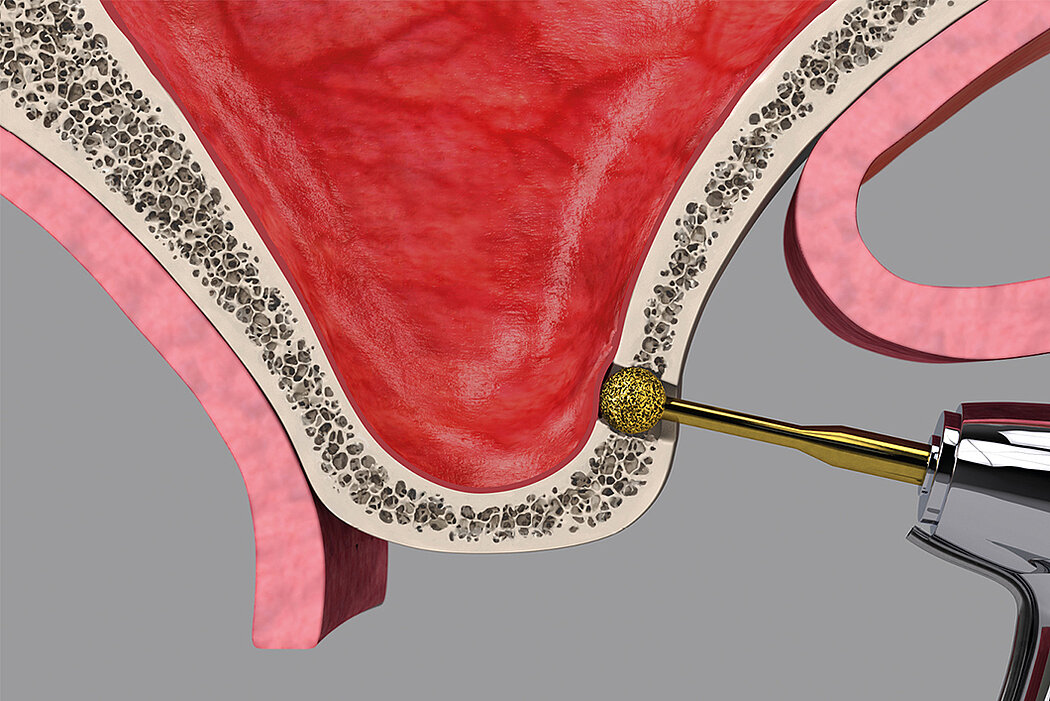
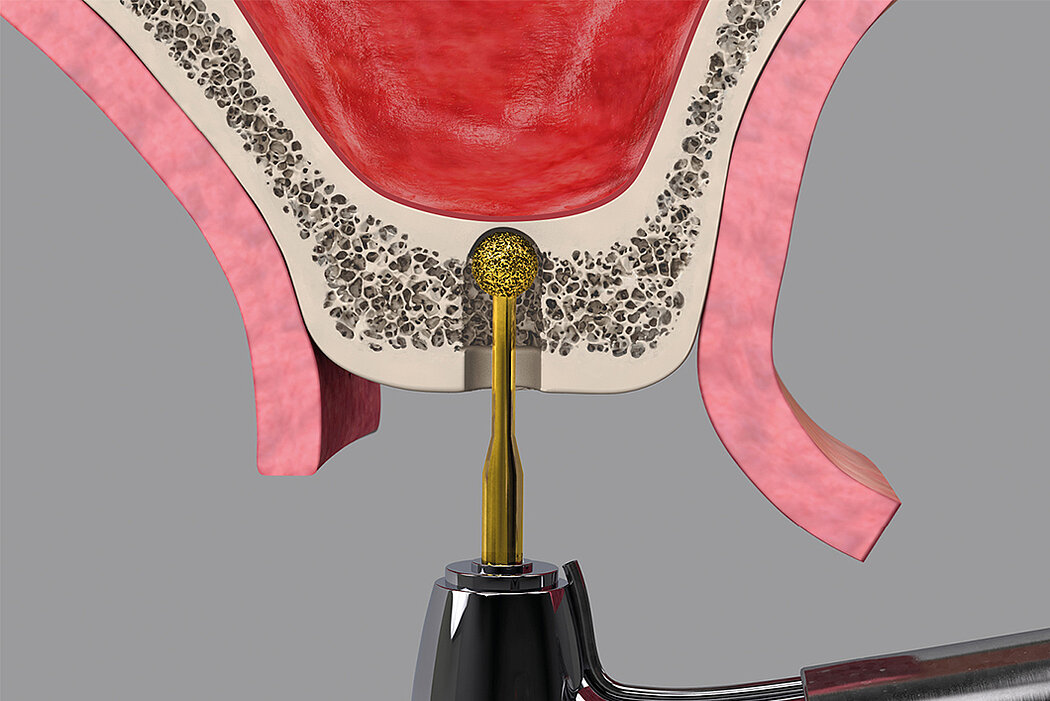
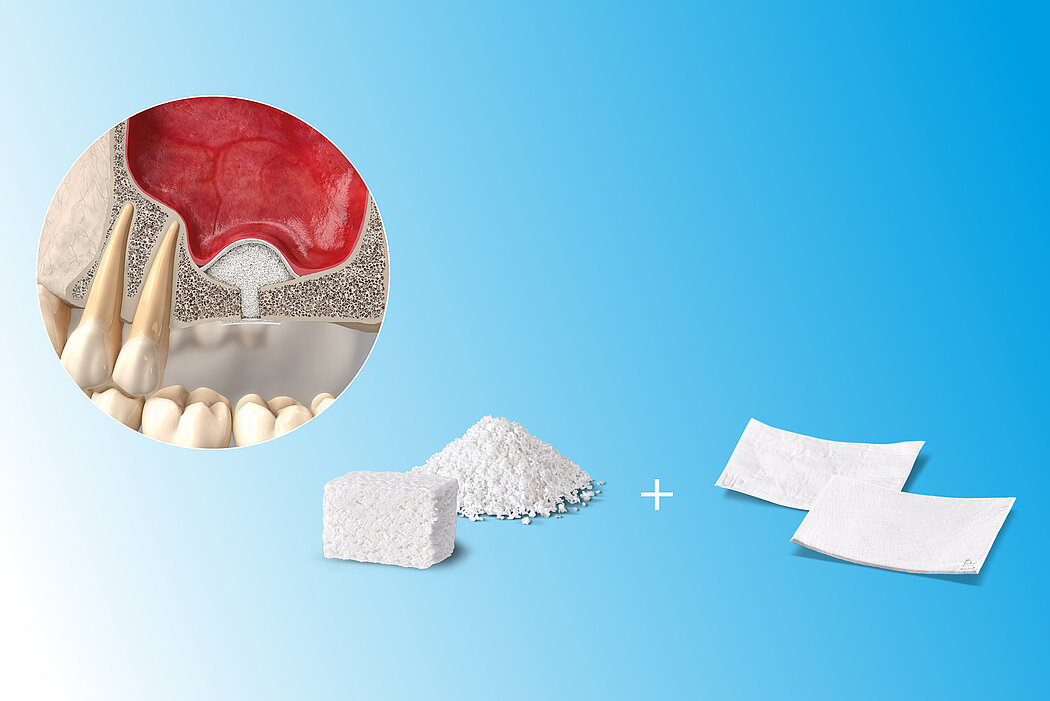
Following the loss of a premolar or molar, posterior maxilla bone height is often insufficient for implant placement. Sinus floor elevation creates more bone height, either via a transcrestal approach or through a lateral window.
Augmentation can be conducted before implant placement (two-stage surgery) or simultaneously (single surgery). Posterior maxilla residual bone and sinus anatomy must be analysed before planning the technique and timing of implant placement. The following table shows a simplified summary of the current guidelines1.
Residual bone height >10 mm (class A)
- Classical implant procedure
Residual bone height 7–9 mm (class B)
- Osteotome technique
- Immediate implant placement
Residual bone height 4–6 mm (class C)
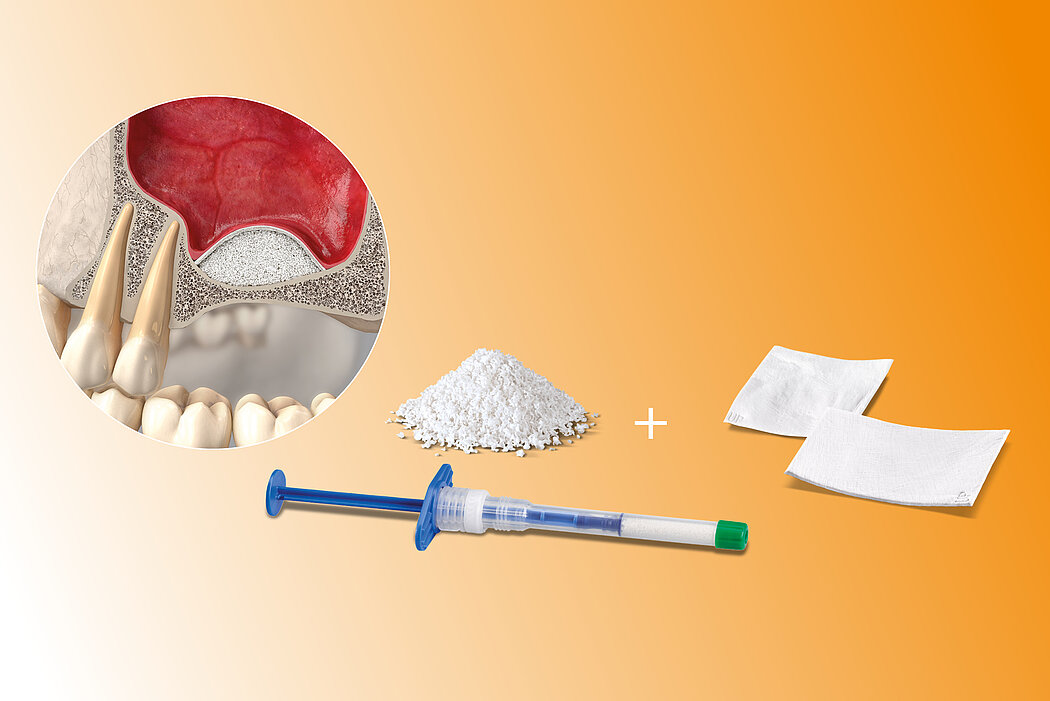
- Lateral antrostomy
- Bone replacement material
- Immediate or delayed implant placement
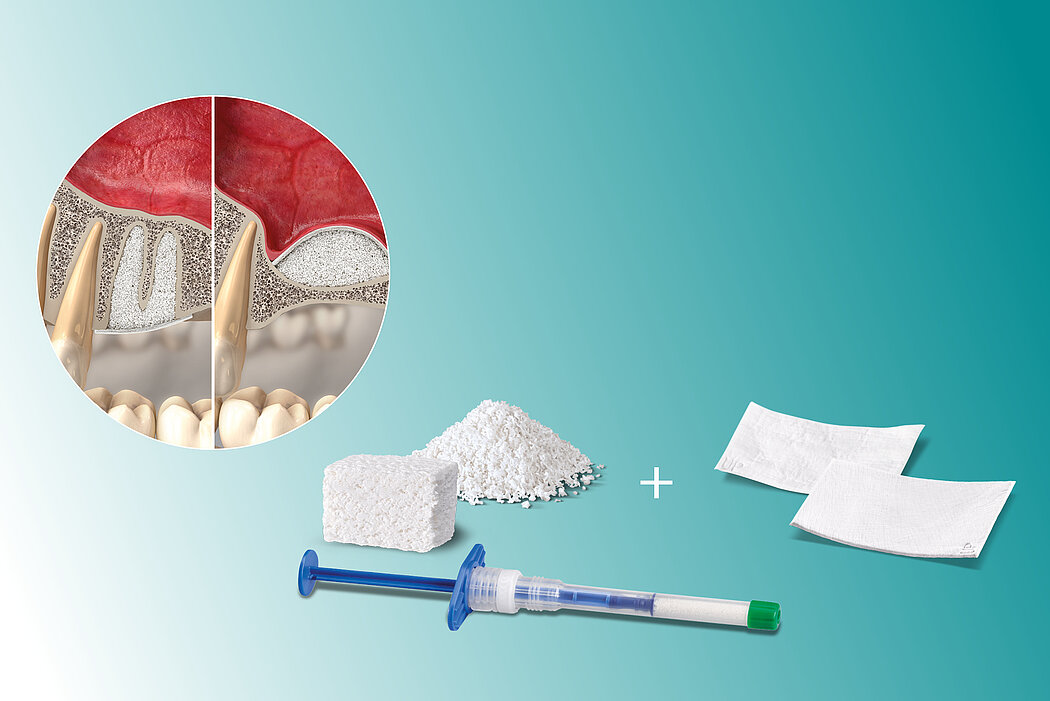
Residual bone height 1–3 mm (class D)
- Lateral antrostomy
- Bone replacement material
- Delayed implant placement
General remark: Immediate implant placement is not recommended with residual bone height <4 mm or poor bone quality.
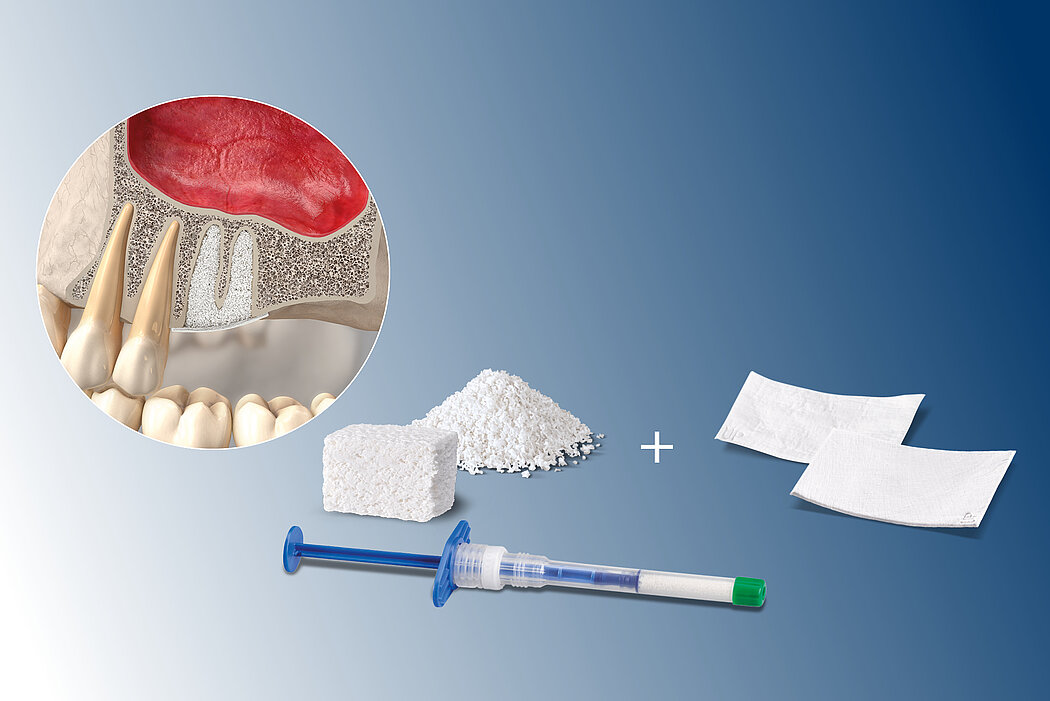
The high reliability of Geistlich biomaterials for these indications has been shown in numerous sinus augmentation studies2-5. Evidence indicates that xenogenous materials produce better long-term results in sinus floor elevation than autogenous bone chips.6
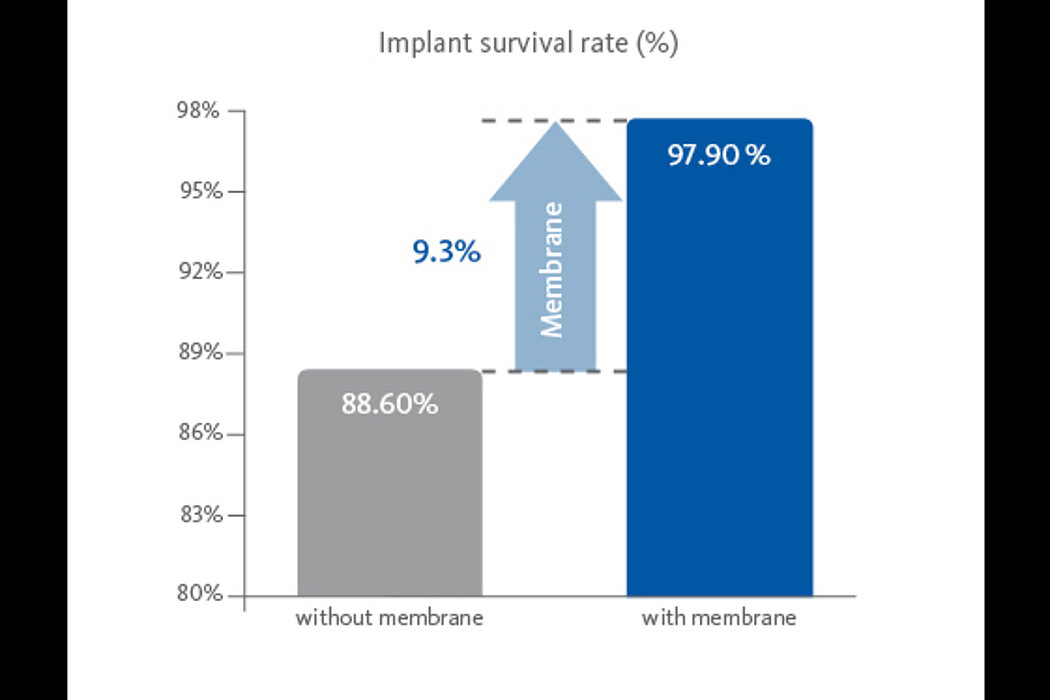
Application of a membrane such as Geistlich Bio-Gide® increases implant survival rate significantly.7 Geistlich Bio-Gide® can also be placed to protect a perforated Schneiderian membrane during surgery and healing.8
References:
- Jensen OT, et al., International J Oral Maxillofac Impl 1998; 13 Suppl: 11-45 (Clinical study).
- Valentini P, Abensur D, Int J Periodontics Restorative Dent 1997; 17(3): 232-41 (Clinical study).
- Valentini P, et al., Int J Periodontics Restorative Dent 2000; 20(3): 245-53 (Clinical study).
- Traini T, et al., J Periodontol 2007; 78(5): 955-61 (Clinical study).
- Valentini P, et al., Clin Oral Implants Res 1998; 9(1): 59-64 (Clinical study).
- Aghaloo TL, Moy PK, Int J Oral Maxillofac Implants 2007; 22: 49-70 (Clinical study).
- Pjetursson BE, et al., J Clin Periodontol 2008; 35 (Suppl. 8): 216-40 (Systematic review).
- Alayan J, Ivanovski S, Clin Oral Implants Res. 2018 Feb;29(2):248-262 (Clinical study).
- Rasperini G, et al., Int J Periodontics Restorative Dent 2010; 30(3):265-73 (Clinical study).
- Weng D, et al., Eur J Oral Implantol 2011 ; 4 (Suppl): 59-66 (Systematic review).
- Jain N, et al., J Clin Diagn Res 2016; 10(9): Ze14-ze17 (Clinical study).
- Palacios JAV, et al., Clin Oral Investig 2018; 22(1): 69-80 (Clinical study).
- Pubmed February 2020. Search term “Bio-Oss” and “Sinus”.
Alternatives to sinus floor elevation
Both Ridge Preservation and short implants can be regarded as alternatives to sinus floor elevation. Ridge Preservation directly after tooth extraction is a minimally invasive approach allowing preservation of >90% of bone volume in the posterior region.9 Following Ridge Preservation, standard implant placement can usually be performed without the need for additional bone augmentation.10
Even though long implants are considered the best option, short implants (<10 mm) can be a valid alternative. Advances in surface geometry and texture have increased the bone-to-implant contact area, leading to improved primary stability and longterm osseointegration.11
However, short implants have certain limitations, such as an unfavorable crown-to-implant ratio, poor aesthetics in the anterior atrophic maxilla and difficult plaque control. In addition, in cases with marginal bone loss, the risk of implant failure is increased due to reduced bone to implant contact.12
More than 300 studies on sinus floor augmentation with Geistlich biomaterials document how well studied Geistlich products are in this indication.13
References:
- Jensen OT, et al., International J Oral Maxillofac Impl 1998; 13 Suppl: 11-45 (Clinical study).
- Valentini P, Abensur D, Int J Periodontics Restorative Dent 1997; 17(3): 232-41 (Clinical study).
- Valentini P, et al., Int J Periodontics Restorative Dent 2000; 20(3): 245-53 (Clinical study).
- Traini T, et al., J Periodontol 2007; 78(5): 955-61 (Clinical study).
- Valentini P, et al., Clin Oral Implants Res 1998; 9(1): 59-64 (Clinical study).
- Aghaloo TL, Moy PK, Int J Oral Maxillofac Implants 2007; 22: 49-70 (Clinical study).
- Pjetursson BE, et al., J Clin Periodontol 2008; 35 (Suppl. 8): 216-40 (Systematic review).
- Alayan J, Ivanovski S, Clin Oral Implants Res. 2018 Feb;29(2):248-262 (Clinical study).
- Rasperini G, et al., Int J Periodontics Restorative Dent 2010; 30(3):265-73 (Clinical study).
- Weng D, et al., Eur J Oral Implantol 2011 ; 4 (Suppl): 59-66 (Systematic review).
- Jain N, et al., J Clin Diagn Res 2016; 10(9): Ze14-ze17 (Clinical study).
- Palacios JAV, et al., Clin Oral Investig 2018; 22(1): 69-80 (Clinical study).
- Pubmed February 2020. Search term “Bio-Oss” and “Sinus”.