Soft-Tissue Regeneration
Scientific background
Soft-Tissue Regeneration plays an increasingly important role in periodontology as well as oral and maxillofacial surgery. Several studies have shown that soft tissue volume and quality not only play a role in esthetics, they are also important for oral health in many respects.1,2
The standard material for soft tissue regeneration is autologous free gingival grafts and connective tissue grafts. However, harvesting those grafts, usually from the palate, is painful, technically demanding, time consuming and may lead to complications such as bleeding, pain, swelling, and occasionally also numbness or infections.3-6
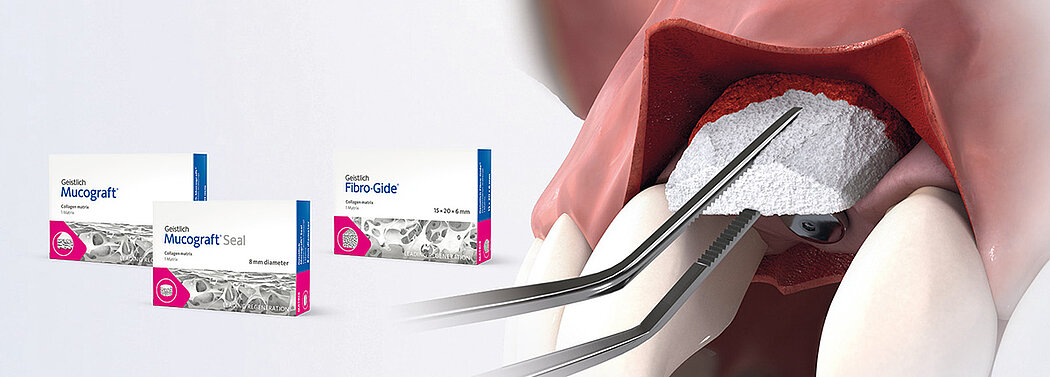
Geistlich's soft tissue substitute product line including Geistlich Fibro-Gide®, Geistlich Mucograft® and Geistlich Mucograft® Seal offer many advantages over autologous grafts:
- No donor site means less pain7,8,9, morbidity3 and surgical chair-time7,8,9,10
- The texture and color of the regenerated tissue match the surrounding native tissue5,6
Geistlich's three collagen matrices are optimized for different applications.
Geistlich Fibro-Gide® is recommended in submerged healing
- for thickening the soft tissue around teeth and implants and under pontics
- to cover recession defects
Geistlich Mucograft® is recommended in open and submerged healing.
- open healing for gaining keratinized tissue around teeth or implants
- submerged healing to cover recession defects
Geistlich Mucograft® Seal is recommended in open healing
- for covering extraction sockets during Ridge Preservation
References:
- Giannobile WV, et al.: Clin Oral Implants Res 2018; 29 Suppl 15: 7-10. (consensus report)
- Perrussolo J, et al.: Clin Oral Implants Res 2018 Oct 22 [Epub ahead of print]. (clinical study)
- Griffin TJ, et al.: J Periodontol 2006; 77: 2070-79. (clinical study)
- Soileau KM, et al.: J Periodontol 2006; 77: 1267-73. (clinical study)
- Zucchelli G, et al.: J Clin Periodontol 2010; 37: 728-38. (clinical study)
- Cairo F, et al.: J Clin Periodontol 2012; 39: 760-68. (clinical study)
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76. (clinical study)
- Geistlich Mucograft® Seal Advisory Board Meeting Report, 2013. Data on file, Geistlich Pharma AG, Wolhusen, Switzerland.
- Thoma DS, et al.: J Clin Periodontol. 2020 Feb 24. doi: 10.1111/jcpe.13271. [Epub ahead of print]. (clinical study)